再生医院

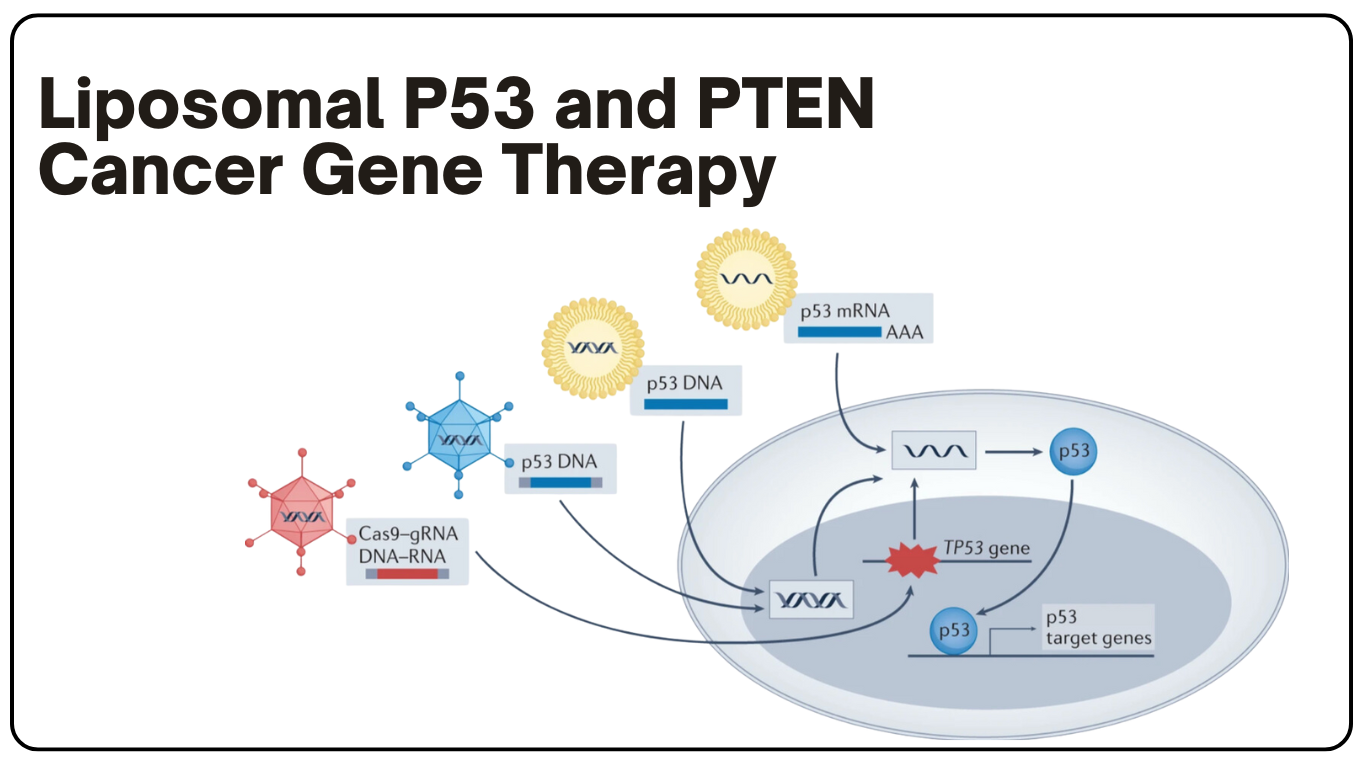
P53和PTEN癌症基因治疗
癌症基因治疗使用的是P53和PTEN肿瘤抑制基因治疗癌症
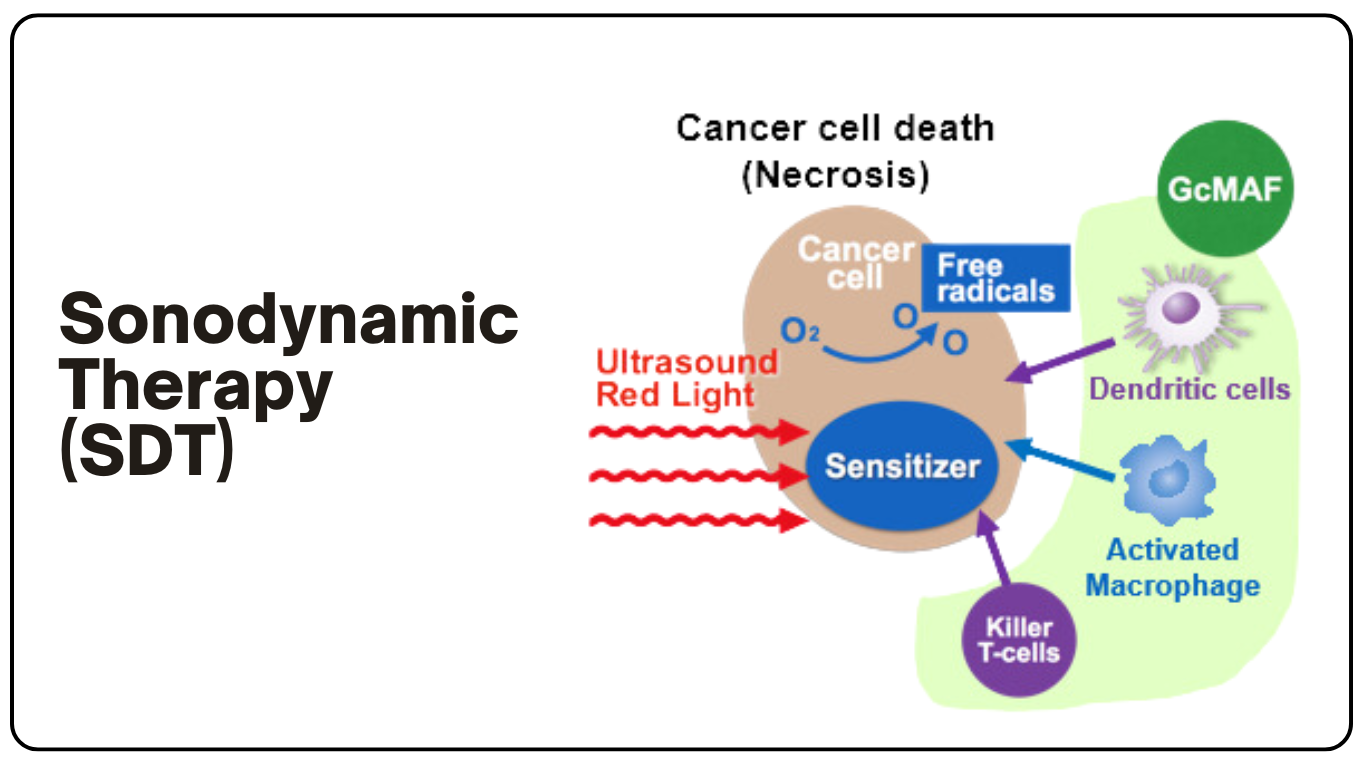
声动力疗法(SDT)
声动力疗法(SDT)是一种非侵入性癌症治疗方法,使用超声波和化学制剂治疗肿瘤

电场疗法 (TTF)
TTF疗法是一种低强度电场疗法 电场抑制体内癌症细胞增殖。

高强度聚焦超声 (HIFU)
国内名称“磁波刀”,是一种先进的治疗方法,将超声能量聚焦在治疗靶点,通过高强度聚焦超声消融病灶达到治疗目的。
News


自闭症人群 (ASD)
ASD的主要因素是什么?ASD与小胶质细胞有何关联?联系我们了解更多关于Saisei ASD改善方法的信息。

癌症治疗
有可能战胜癌症吗?了解Saisei的尖端技术,帮助您的免疫系统对抗癌症。

Saisei Clinics
Saisei Clinics is a pioneering medical clinic that leverages innovative technologies and the latest advancements in medicine through collaborations with leading Japanese and international research institutes and companies. We proudly hold patents for several unique technologies and products designed to enhance natural immunity and rejuvenate the entire body at the cellular level.

在长寿和恢复活力方面的突破性发现!
MAF对长寿和抗衰老的影响进行了临床研究。结果令人震惊!联系我们了解更多详细信息。
While we acknowledge traditional methods of treatment, our primary goal is to assist patients in overcoming various disorders and dysfunctions by harnessing their innate immunity and promoting internal rejuvenation. We focus on normalizing the natural vital immunity of individuals. In addition to immunotherapy, our clinic has developed a cutting-edge form of gene therapy known as liposomal gene therapy, recognized for its exceptional effectiveness and safety.

At our clinic, each patients are highly valued, and we tailor our approach to provide an individualized combination of treatment methods based on their medical history, predispositions, and current condition. Our guiding principle is “FIRST DO NO HARM.” Our treatments are grounded in scientific research, ensuring safety and minimal side effects. Even if patients are undergoing traditional medical treatments, they can confidently incorporate our methods as complementary therapies.
For more information about our treatment methods, please scroll below.

At our clinic, each patients are highly valued, and we tailor our approach to provide an individualized combination of treatment methods based on their medical history, predispositions, and current condition. Our guiding principle is “FIRST DO NO HARM.” Our treatments are grounded in scientific research, ensuring safety and minimal side effects. Even if patients are undergoing traditional medical treatments, they can confidently incorporate our methods as complementary therapies.
For more information about our treatment methods, please scroll below.


Dr. Toshio Inui
CEO of Saisei Mirai Clinic group
日本医院及场所
我们在东京、大阪、神户有三个诊所及一间生物实验室
大阪癌症治疗中心

神户抗衰诊所

东京科学诊所

细胞培养中心

再生未来联系方式
- 日本客服电话:+070-1834-8866;中国客服电话:+86 13720897727;微信号码:LinkHealth520
- 中国市场官网: https://www.91linker.com/
海外分支机构

Saisei Hawaii

Saisei Australia

Saisei Lithuania

Saisei Serbia

Saisei Spain

Saisei Sweden

再生制药公司
再生制药公司是一家于2014年在德岛大学成立的生物医药公司。
我们开发、研究和制造免疫激活食品“MAF系列(胶囊、喷雾、糖果、粉末)”、超声波增敏剂和电场疗法(TTF、ECCT)等。
我们为国内外客户提供我们的免疫激活食品“MAF系列”。
我们现在位于大阪,我们的产品在大阪的一家GMP认证工厂生产。
作为Saisei Mira集团,我们与每个诊所合作,并将继续我们的研发,旨在治疗许多疾病,如急性感染、慢性感染、癌症、自闭症、慢性疲劳综合征、多发性硬化症、类风湿性关节炎、阿尔茨海默病、痴呆症等。

Coming soon!
Opening of clinic in Lithuania!
For additional information, please contact us or refer to the Saisei Lithuania website below.
Opening of clinic in Lithuania!
For additional information, please contact us or refer to the Saisei Lithuania website below.
We are pleased to announce the opening of our clinic in Serbia.
For additional information and inquiries, please feel free to contact us using the details below.

You are invited to a Zoom webinar.
When: Oct 22, 2023 10:00 AM Osaka, Sapporo, Tokyo
Topic: Saisei Medical Conference 2023 -Advances in Immunotherapy and Rejuvenation: Enhancing Health and Longevity
Register in advance for this webinar:
https://us06web.zoom.us/…/reg…/WN_YJIRkF2cSA6iPZb7b4HCwg
After registering, you will receive a confirmation email containing information about joining the webinar.

read more
CLINICAL STUDY MAF capsules efficacy in post-COVID syndrome treatment is started in KAZAKHSTAN
MedInc Ltd in Kazakhstan on the base of National Research Cardiac Surgery Center has started the implementation of prospective randomized open-label clinical trial: “Evaluation of immune modulator MAF capsules efficacy in a therapy of patients with post-COVID syndrome”. The study sponsored by the developer and manufacturer of the study product Saisei Pharma Co. Ltd, Japan.
The goal of the study is the evaluation of the safety and efficiency of a MAF capsules dietary supplement with anti-inflammatory immune response modulating function in combined treatment of the post-COVID syndrome. |
The trial started in February 2021 and targeted 200 patients with post-COVID syndrome, which will be randomized 1:1 in two groups:
Group 1: SOC (standard of care) + dietary supplement MAF capsules administration for 30 days.
Group 2: SOC
The study primary endpoints aim to evaluate the improvement within 30 days period after MAF capsules administration, where the clinical efficacy refers to an improvement of life quality according to Chalder Fatigue Scale and the “Postcovid syndrome” consequences and symptoms severity evaluation according to ISARIC questionnaire.
The study secondary endpoints included the evaluation of changes according to the Karnofsky Performance Scale and Numerical Rating Scale, and evaluation of the MAF capsules side effects.
The exploratory endpoints include effects on:
1. Lymphocyte count, T cells and its subpopulation count, and B cells count
2. The plasma level of IgG against SARS-CoV-2
read more
- Control group
- MAF Capsules (colostrum MAF) group
- M Capsules (whey MAF) group
- SOC (standard of care)
- SOC plus MAF Capsules (148 mg, 3 caps. TID for 14 days)
- SOC plus M Capsules (148 mg, 3 caps. TID for 14 days)
- No adverse events
- Decrease in the mortality rate
- Decrease in necessity and duration of supplemental oxygen
- Decrease in time to recovery
- Decrease in time until hospital discharge
- Preventing of respiratory failure
- Restoring the base-line decreased lymphocytes count
第三代GcMAF是由我们拥有专利的再生制药公司开发的。一家在香港注册的公司在澳大利亚网上销售假冒GcMAF产品。请注意,第三代GcMAF产品仅由再生制药生产。其他公司的任何其他产品都是假冒产品。
News
We successfully concluded the medical conference! A big thank you to all the doctors who presented.
https://saisei-pharma.co.jp/maf-capsulestriple-en/
The hypothesis: Based on the aforementioned findings and on documented analogies between SARS-CoV-2 and HIV, we hypothesized that the reduced conversion activity of the Gc protein (human group-specific component (Gc)) into the macrophage activating factor (MAF) could have a key role in the dysregulate immune response induced by SARS-CoV-2, just like for HIV infected patients. If this hypothesis is correct, it might help to set a valid strategy of immunotherapy also based on an off-label use of GcMAF in critically ill COVID-19 patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7513798/
Conclusion: According to the provided literature overview, we firmly believe that GcMAF deserves be tested as immune-therapeutic to increase macrophages functionality for earlier SARS-CoV-2 viral control, protection against COVID-19 progression by limiting epithelial damage, control local inflammation and prevent from the hyperinflammatory immune response. For this purpose, we planned a Phase-II interventional clinical trial evaluating the effectiveness and safety of Oral immunotherapy with Third Generation GcMAF in hospitalized patients with COVID-19 pneumonia (COral-MAF1 Trial) at the “Ospedale del Mare” Hospital, Naples, Italy.
Saisei Pharma plans clinical study of oral MAF in COVID-19 patients details were featured on some website.
Please check the below URL.
This time, the Saisei Mirai, has contracted official partnership with the Ukrainian National Cancer Center as partner hospitals.
[smartslider3 slider=”2″]